
By Anthony Dimitrokalis
Translation & Localization Solutions Specialist
Navigating International Expansion in the Heavily Regulated Medical Device and Pharma Industries

A day in the life of many pharma and medical device professionals.
Those of us who have worked in pharmaceutical, biotech, or medical device facilities/plants have a responsibility to detail and quality that few others have. After all, people’s lives depend on it. As an employee in these facilities, you know how much painstaking effort you take and how many long hours you work to ensure the product and supporting documentation are perfect, or at least devoid of too much red ink.
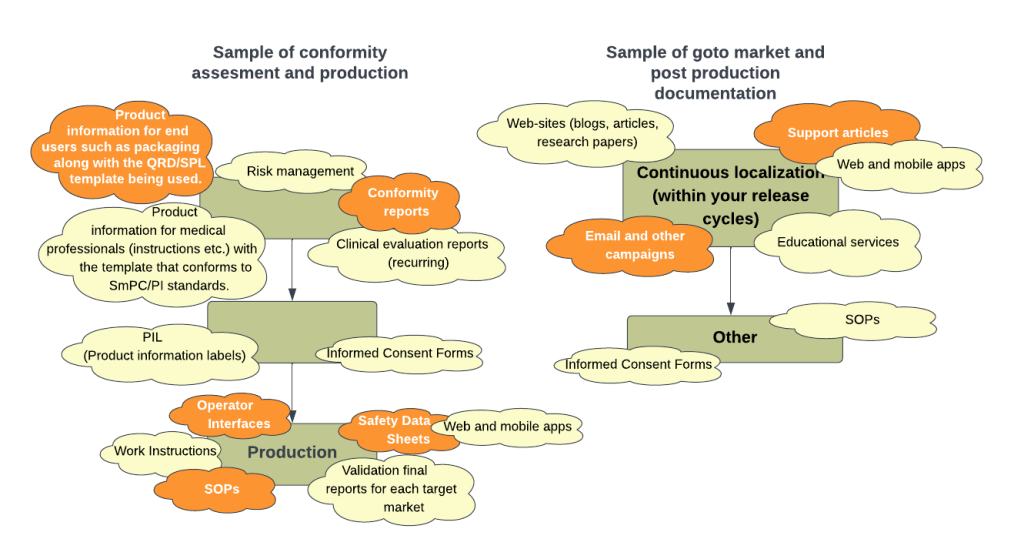
Sometimes it feels that for every hour of work, one day’s worth of supporting documentation is required, which makes it time-consuming to determine which documents are relevant for foreign markets. The diagram in the header outlines some of the constraints and document types these industries deal with.
Challenges when it comes time to test, produce, or sell into International markets
Any individual company in these industries can fill a decent-sized public library with printed and electronic documents that can be potentially translated, making costs a considerable constraint. This has to be balanced with quality, confidentiality, accuracy, cultural relevance, and appropriateness for global regulatory agencies and consumers.
The complexity increases with Clinical Outcome Assessments (COAs) and assessment questionnaires, which can require the equivalent of a focus group in layperson’s terms simply for testing translations, let alone multiple QA iterations (linguistic validation and cognitive debriefing).
Auditors are required for validation report translation, and then there are the work instructions that factory workers in different countries rely on.
Choosing a translation vendor
When choosing a translation vendor, ensure they take the same meticulous care when performing translations that the employees and contractors who created the source documents took.
Check for the following:
- Confirm that the vendor has an ISO 17100:2015 certification for quality and an ISO 27001:2013 for data protection.
- Verify their ability to have multiple review processes with qualified subject matter experts.
- Ensure that each step in the review process is documented and traceable.
- Confirm and agree to a QA process and final QA metrics and reports that can be easily tracked on the end user side.
- Avoid machine-translated or AI-driven processes for the most confidential of documents and those that regulatory agencies verify.
- Find an agency with trained personnel and native speakers in multiple aspects, from localization, linguistic validation, and source content validation (e.g., validation reports, OQ, IQ, etc.). These agencies provide the highest quality solutions.
- Ensure that glossaries and TMs are updated to implement standards and reuse of already validated and approved translations. This also lowers costs significantly.
- For glossaries and translation memory, consider agencies that use AI enhancements that further enforce standards while improving translation quality and lowering costs while maintaining confidentiality.
- Only some can translate all required documents well; therefore, find agencies to handle your specific document type. The documents that GTH translation handles are indicated in the image below:
Try out our FREE localization QA audit!
Don’t forget to accept our offer of giving you a free localization QA audit! We’ll test your website to see if it passes all of our localization checks, then provide you with an audit on just how localized your website is.
Simply click here https://gthtranslation.com/lqa-services, fill in your details at the bottom of the page, and we’ll do the rest! The LQA audit is vital as it will show you if you’re properly communicating with your clients or customers (in different geos).



 English
English Greek
Greek +44 730 8577 353
+44 730 8577 353 +357 25 55 42 10
+357 25 55 42 10